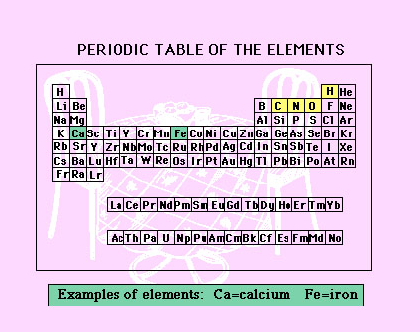
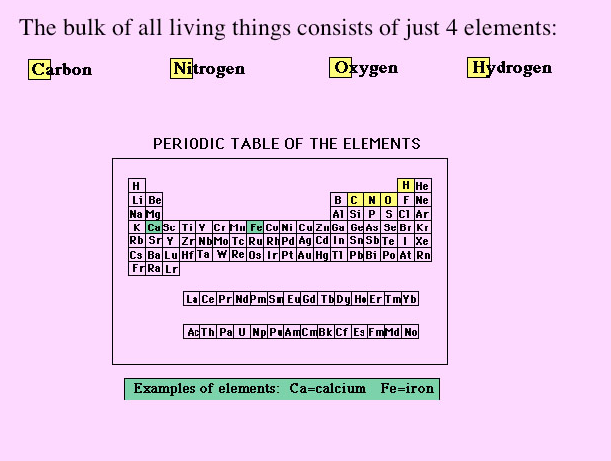
| Now For the Lecture... The first part of lecture 4-1 and the study questions for this week (#1-12) will focus on introducing you to atoms, electrons, bonds, and molecules. I have a few different resources to help explain these abstract concepts below.
|
Why is this information important? Throughout this course we will be looking at the chemical structure of nutrients. The purpose of these resources is to introduce you to chemical structures, and explain what elements, electrons, and covalent bonds are. "The Path to Inner Peace" explains these concepts in a very simple, visually appealing way. |

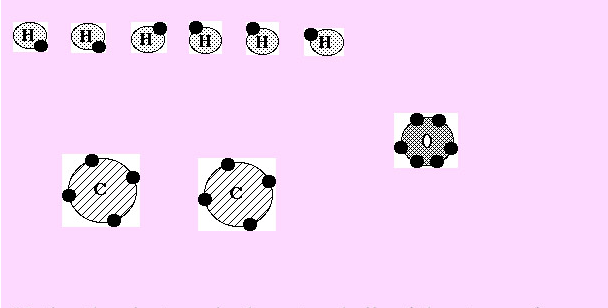
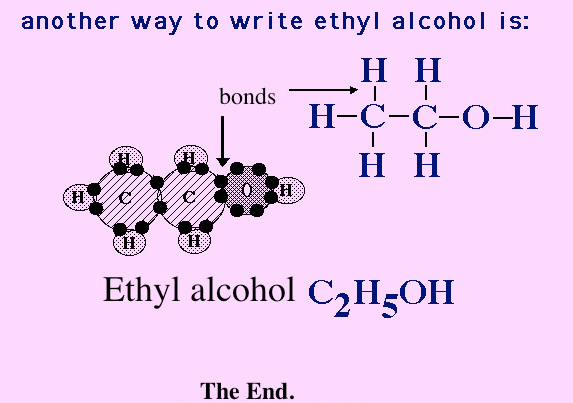
Below are the elements that make up ethyl alcohol. Notice they do not have full outer shells. Therefore, they will go hunting to bond with other elements to complete their outer shell.
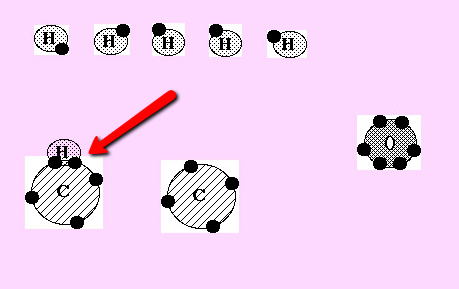
Can you see that one of the 6 hydrogen atoms in the picture above has now moved down to combine with a carbon atom? This hydrogen would now have a full outer shell (2 electrons). The carbon still does not have a full outer shell with only 5 electrons. Remember carbon needs 8 electrons in its outer shell to be full and stable.
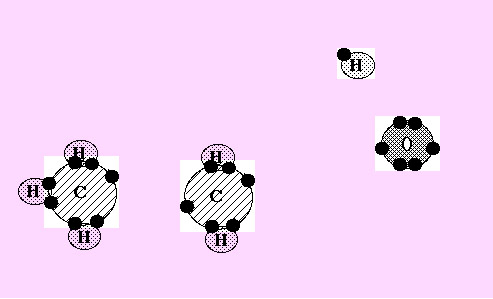
Now 5 of the 6 hydrogen atoms in this picture have moved down to combine with carbon atoms.
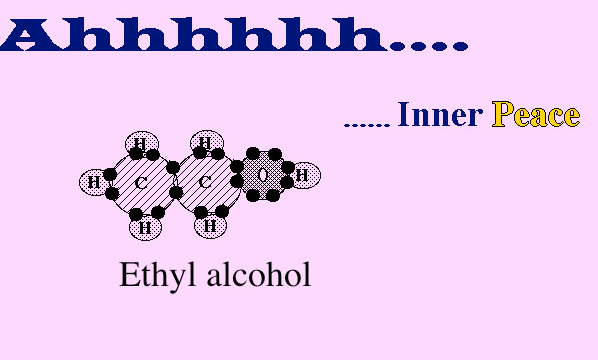
Once all the carbon, hydrogen and oxygen bond...
Inner peace because all the atoms have full outer
shells. Hydrogen-2, oxygen- 8 and carbon-8. There is energy in these
covalent bonds, and we can utilize this energy when we break these
bonds in cells. More to come on that in lecture 3B.
The youtube video below is another resource to introduce you to atoms, electrons, and molecules. This video will give you slightly more detail and will contain more animation.
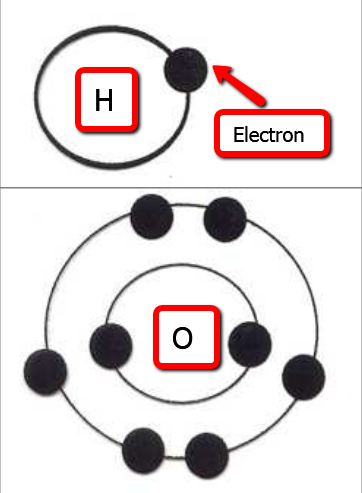
Here is a link to the above video: http://youtu.be/eESpP5okA1I The video mentions fat and carbohydrates being the energy providing nutrients. From week 1 you also know that protein is an energy-yielding nutrient, but it is not the main role of protein (perhaps that is why it is not mentioned). In addition to Carbon, Hydrogen, and Oxygen, proteins also contain Nitrogen. The above video discusses atoms needing 8 electrons to fill their outer shell, like carbon and oxygen. What is not mentioned is that Hydrogen only has one valence shell, so it only needs 2 electrons to have a full outer shell and be stable. In the lecture outline you will see the following images. The first image is representing Hydrogen, which only has 1 valence shell, and only has 1 electron, so it is looking to bond with another atom to complete its outer shell with 2 electrons. Oxygen has two shells, the first one already being full, and the second one only having 6 electrons, so oxygen needs 2 more electrons to have a full outer shell of 8.
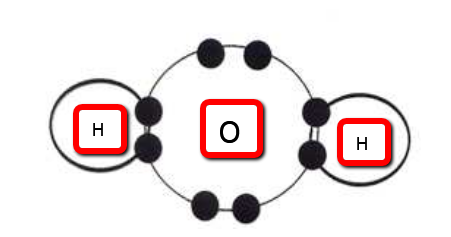
When these atoms come together to make the molecule, water (H2O), as seen below, they both are happy and stable, with hydrogen now having a full outer shell of 2 electrons (through the sharing of electrons, or covalent bonding), and oxygen having a full outer shell with 8.
|