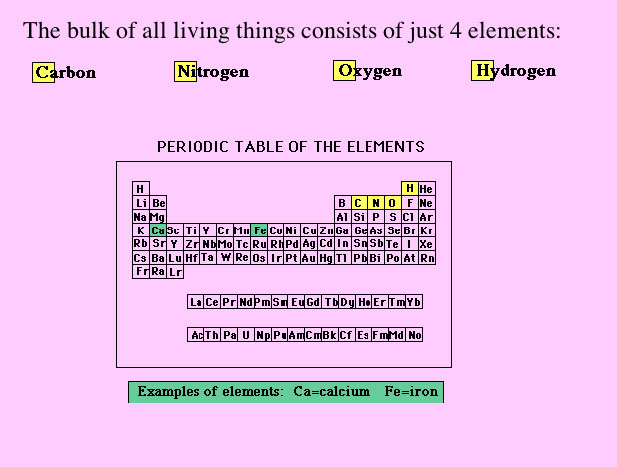
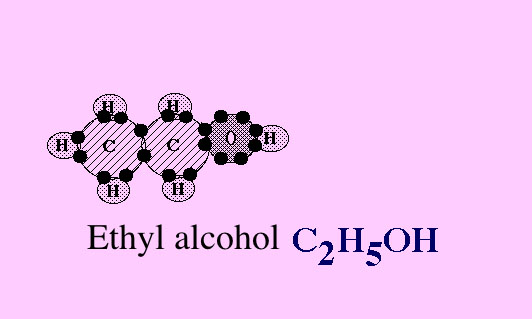(We are now the Health Professions Division.)










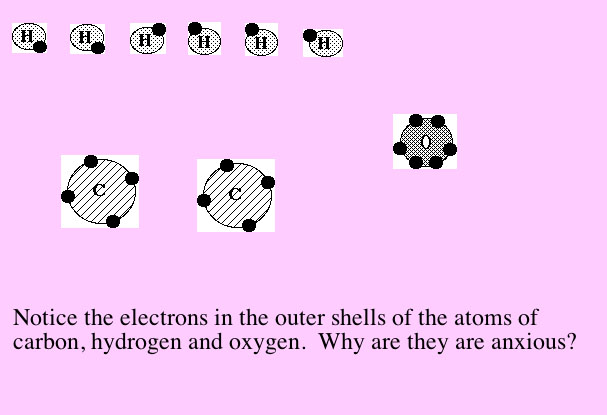
| What it looks like with
motion: |
| - |
Now let's look at it again without the motion.
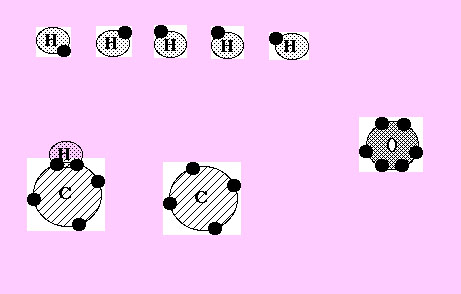
Can you see that one of the 6 hydrogen atoms in the picture above has now moved down to combine with a carbon atom.
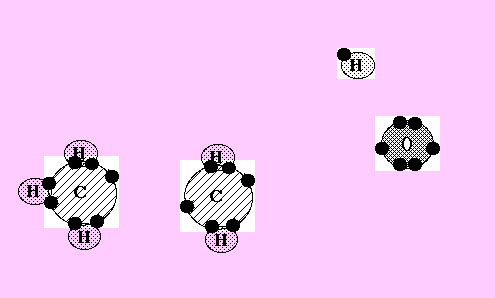
Now 5 of the 6 hydrogen atoms in this picture have moved down to combine with carbon atoms.
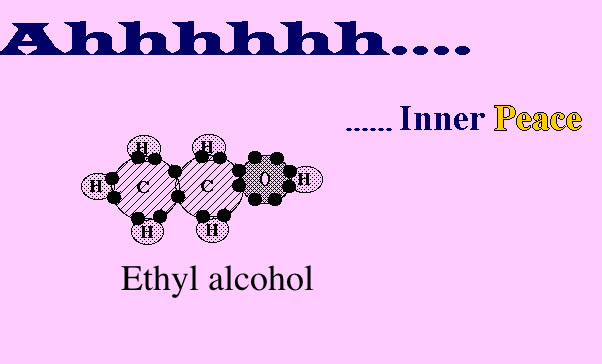
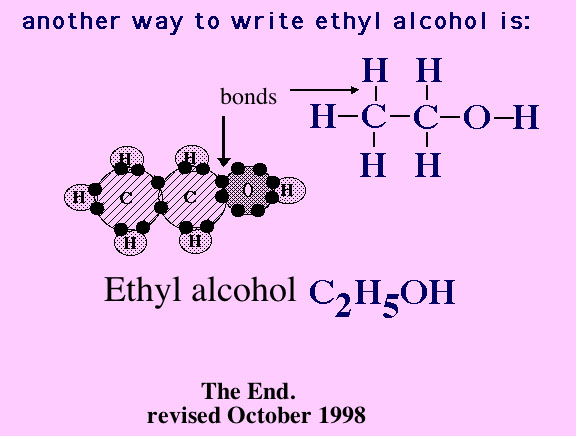
Now the oxygen atom has joined with atoms of carbon and hydrogen, forming ethyl alcohol.
The sun's energy is now in the bonds between the atoms, especially in the carbon to carbon bonds.